Experience Matters
Providing expert advice in health economics, pricing & reimbursement, market access and regulatory affairs in Australia.
Welcome to Lucid Health Consulting: Your Gateway to Pharmaceutical and Medical Markets in Australia and the Asia-Pacific
With over 30 years of industry leadership, Lucid Health Consulting is the trusted partner for companies and associations in the pharmaceuticals, medical device, medical technology, and biotech sectors in Australia.
Our team of experts includes health economists, regulatory affairs specialists, reimbursement and HTA consultants, market and patient access advisors, and statisticians. We have a proven track record of enabling companies and organisations to optimise the entry of their pharmaceuticals, biotech products medical devices and technologies into the Australian market.
Whether you’re looking to overcome complex regulatory hurdles, answer critical business questions, streamline your market entry process or require product sponsorship in Australia, we are here to help.
Let us help you unlock new opportunities and drive success across Australia and the Asia-Pacific region.
How we can help your business?
Unlock the potential of the Asia-Pacific Market with Lucid Health Consulting
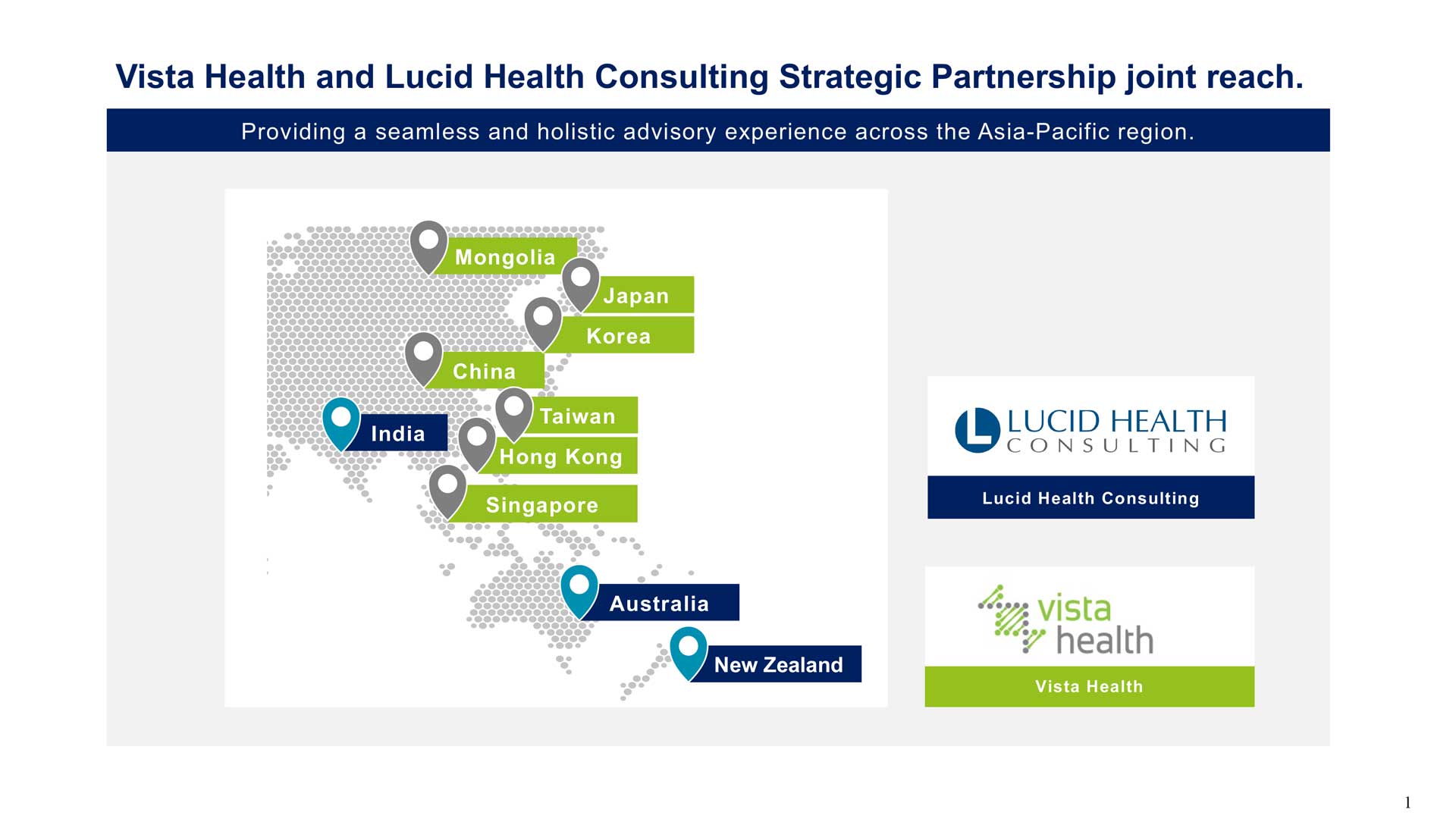
Let us help facilitate your pathway to successful approval and launch of your pharmaceuticals, biotech products, medical devices, and technologies across the Asia-Pacific Region through our strategic partnership with Vista Health , headquartered in Singapore, with offices in Japan and the UK.
Together, we leverage our combined expertise and expansive network across the region to offer tailored advice, strategic insights, and seamless implementation, paving the way for your successful market entry across the Asia-Pacific region.
What’s New
Read our articles, find out about our upcoming webinars and speaking engagements for the latest industry insights.
Unlocking the Power of Social Media for Real-World Evidence in Healthcare
Unlocking the Power of Social Media for Real-World Evidence in Healthcare Our Senior Consultant of Regulatory Affairs at Lucid Health Consulting, Kathryn Williams Day, offers insightful commentary on a recent abstract published in Springer Link. Titled “Using...
Co-design of an Enhanced Consumer Engagement Process for health technology assessment in Australia.
Co-design of an Enhanced Consumer Engagement Process for health technology assessment. The Enhanced Consumer Engagement Process is one of the key commitments in the 2022-2027 strategic Agreements with Medicines Australia and the Generic and Biosimilar Medicines...
Lucid Health Consulting: NEWSLETTER #1 – February 2024.
Read our first newsletter of 2024, "2024 - The Australian Healthcare Market Outlook" as we share our key projections for 2024, recap highlights from 2023, spotlight upcoming Australian and Global Industry events we’ll be attending, and unveil useful online resources,...
What our clients are saying.
Lucid Health Consulting are providers of expert advice in health economics, pricing & reimbursement, market access and regulatory affairs in Australia. Enabling companies to optimise the entry of their pharmaceutical, biotech and medical devices into the Australian market.











